Novel Molecular Recognition Principle via van der Waals Interactions between Nonpolar Portions of VOCs and Polar Solid Surfaces
A research group led by The Univ. Tokyo (Mr. Yusuke Tonomoto, Prof. Wataru Tanaka, Prof. Takeshi Yanagida), RIES Hokkaido Univ., IMCE Kyushu Univ. (Prof. Hikaru Saito) and The Univ. Osaka (Prof. Wataru Mizukami) discovered the critical role of van der Waals interactions between nonpolar portions of volatile organic compounds (VOCs) and polar solid surfaces on the molecular adsorption behaviors on the metal oxide surface.
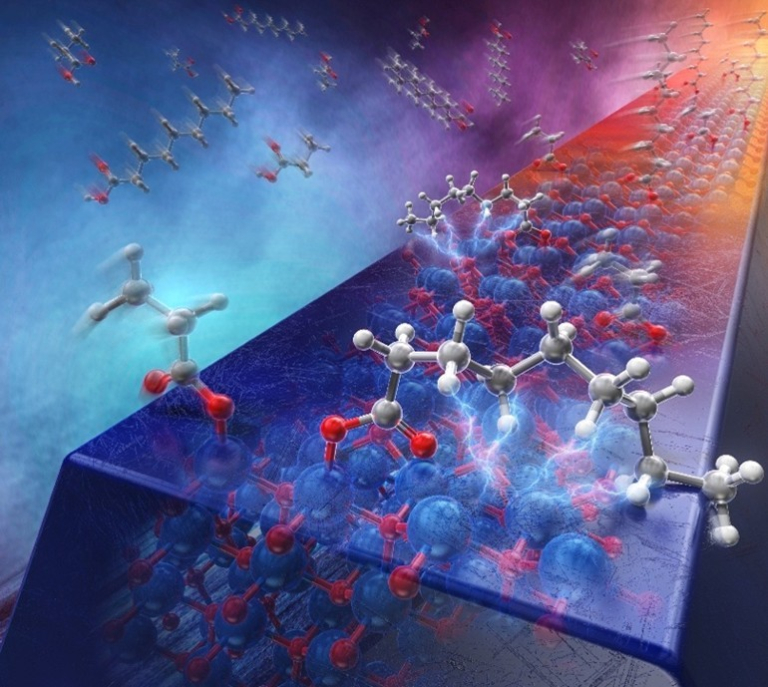
Traditionally, a molecule-to-surface interaction on metal oxide sensor surface has been thought to be solely dominated by interactions between the polar functional group and the polar oxide surface. In this study, we demonstrated a vital role of van der Waals interactions between the nonpolar alkyl chains and the polar ZnO surfaces on molecular adsorption of aliphatic carboxylic acids. Experiments and DFT calculations revealed that the cumulative effect of individually weak van der Waals interactions between nonpolar C–H groups and polar ZnO surfaces becomes the major contributor to adsorption energy for longer-chain carboxylic acids. This finding opens avenue to discriminate the carbon-chain length of volatile aliphatic carboxylic acids via controlling the operation temperature of metal oxide sensors.
The paper has been published in ACS Nano on January 16.
Information of the paper
Title
Van der Waals Interactions between Nonpolar Alkyl Chains and Polar ZnO Surfaces in Gas Sensing Dynamics of Aliphatic Carboxylic Acids
Authors
Yusuke Tonomoto, Wataru Tanaka, Kazuki Nagashima, Takuro Hosomi, Tsunaki Takahashi, Jiangyang Liu, Haruka Honda, Hikaru Saito, Wataru Mizukami, and Takeshi Yanagida
Journal
ACS Nano
DOI
URL
Laboratory of Interactive Functional Materials
URL https://sites.google.com/view/nagashima-lab/











