Dr. Nobuyuki Tamaoki, Professor, and P. K. Hashim, Graduate, Research Institute for Electronic Science, Hokkaido University have directly bonded a central chirality and geometric isomerism, which were considered to be absolutely different molecular structure elements, successfully controlling the generation and loss of a molecular chirality by light.
Flexible control of molecular chirality (that property by which the form of a molecule cannot be superposed on its mirror image) has been a chemists’ dream since Pasteur. In particular, considering the basic issue of how molecules in nature accomplish a single enantiomer molecular system, and the application of chiral molecules to polarization control materials, it is important to elucidate how chirality is changed in response to stimuli.
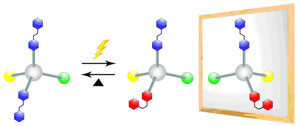
The chirality becomes inherent in the presence of one of the asymmetries in a molecule: the central, axial, plane, or helical chiralities. Of the asymmetries, the central chirality is seen when one carbon has four different substituents. In the past, to dynamically generate or lose the central chirality, the bond between the carbon and the substituent was only cut or recovered. Our research group has synthesized a methane derivative, called azobenzene, that induces two substituents, and we have controlled the geometric isomerism of the azobenzene using light, instead of cutting or recovering the chemical bond, thereby succeeding in the flexible control of the generation and loss of the central chirality. In the future, circularly polarized light, which serves as a chiral template, will be applied to this synthesized chemical compound and related compounds, possibly selectively generating not only a central chirality but also an enantiomer.
Our study is expected to present a new methodology for the control of the chirality that is a basic property of a molecule, and also to indicate a mechanism for the formation of a single enantiomer molecular system of organic chemical compounds in nature, and the possibility of polarization control materials.
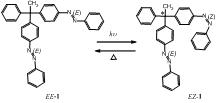
The outcome of our research was published in the electronic version of ‘Angewandte Chemie International Edition, Early View on November 4. "Induction of Central Chirality by E/Z Photoisomerization", P. K. Hashim, Nobuyuki Tamaoki, Angew. Chem. Int. Ed., DOI: 10.1002/anie.201104614









