Assoc. Prof. Chun-Biu Li and Prof. Tamiki Komatsuzaki in the Laboratory of Molecule & Life Nonlinear Sciences, along with Prof. Hiroyuki Noji and his staffs in the Single Molecule Biophysics Laboratory in the University of Tokyo, have successfully uncovered the functional role of the hydrolysis of adenosine triphosphate (ATP), a fundamental reaction in the motor protein F1-ATPase, as a "key" to "unlock" the subsequent reactions in the catalytic cycle.
Driven by ATP hydrolysis, F1-ATPase (F1) is a rotary motor protein that can efficiently convert chemical energy into mechanical work of rotation via fine coordination of its conformational motions and reaction sequences. Extensive studies have been carried out to unveil the tight chemo-mechanical couplings between the rotation and the fundamental catalytic reactions, namely, ATP binding, adenosine diphosphate (ADP) release, bound ATP hydrolysis and inorganic phosphate (Pi)-release. Compared with reactant binding and product release, it was found that the hydrolysis of bound ATP has relatively insignificant contributions to the torque generation and the overall chemical energy released. Therefore it remains elusive what functional role the hydrolysis of bound ATP can play in the F1 catalytic cycle.
In this work, we investigate the detailed kinetics of the catalytic dwells and scrutinize the possible roles of the ATP hydrolysis reaction in terms of contemporary time series analysis and single F1 rotary observations with microsecond time resolutions. In particular, model-free change point and clustering analyses are developed and applied to the stepwise F1 rotary traces in order to robustly construct the statistics of waiting time and angular fluctuations from the catalytic dwells. This allows us to detect a small angular increment during the catalytic dwell triggered by the hydrolysis of bound ATP that is indiscernible in previous studies using conventional analysis methods. Moreover, we find in freely rotating F1 that the hydrolysis of bound ATP is followed by Pi-release with low synthesis rates.
In terms of free-energy reaction diagrams accounting for the chemo-mechanical couplings between the catalytic reactions and rotary fluctuations at the catalytic dwell, we uncover the functional roles of the hydrolysis of bound ATP as a "key" to "kinetically unlock" the subsequent Pi-release reaction and promote the correct reaction ordering, despite its minor contributions to the torque and chemical energy generations. This result provides an important step toward the understanding of the working principles of F1 at the single molecule level. Furthermore, we expect that such key-and-unlock mechanism could be a common strategy to achieve precise chemo-mechanical coordination in other motor proteins, such as V-ATPase, kinesin, myosin, dynein, etc., in the highly fluctuating nano-environments.
This research was conducted in collaboration with the research group of Prof. Hiroyuki Noji (Department of Applied Chemistry, Graduate School of Engineering, University of Tokyo) and was published in Nature Communications on Dec 17th 2015.
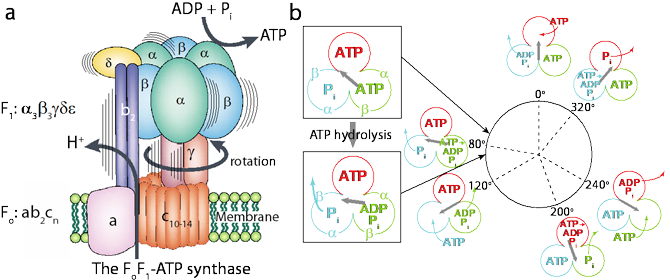
Figure: The FoF1-ATP synthase and the main results of this study. (a) FoF1-ATP synthase composes of the Fo motor with subunits ab2cn and the F1 motor with subunits α3β3γδε. Driven by ATP hydrolysis, an isolated F1 can efficiently convert chemical energy into mechanical work of rotation. (b) The chemo- mechanical coupling scheme of the F1 catalytic cycle as a function of the angle of g-subunit. Colored circles show the nucleotide states. Main results from current study are shown inside the boxes: ATP hydrolysis triggers a small rotation of the g-subunit, which can kinetically assist the subsequent release of Pi.










